Medical Device Complaint Management Market: Insights and Competitive Analysis
"Executive Summary Medical Device Complaint Management Market Research: Share and Size Intelligence
CAGR Value
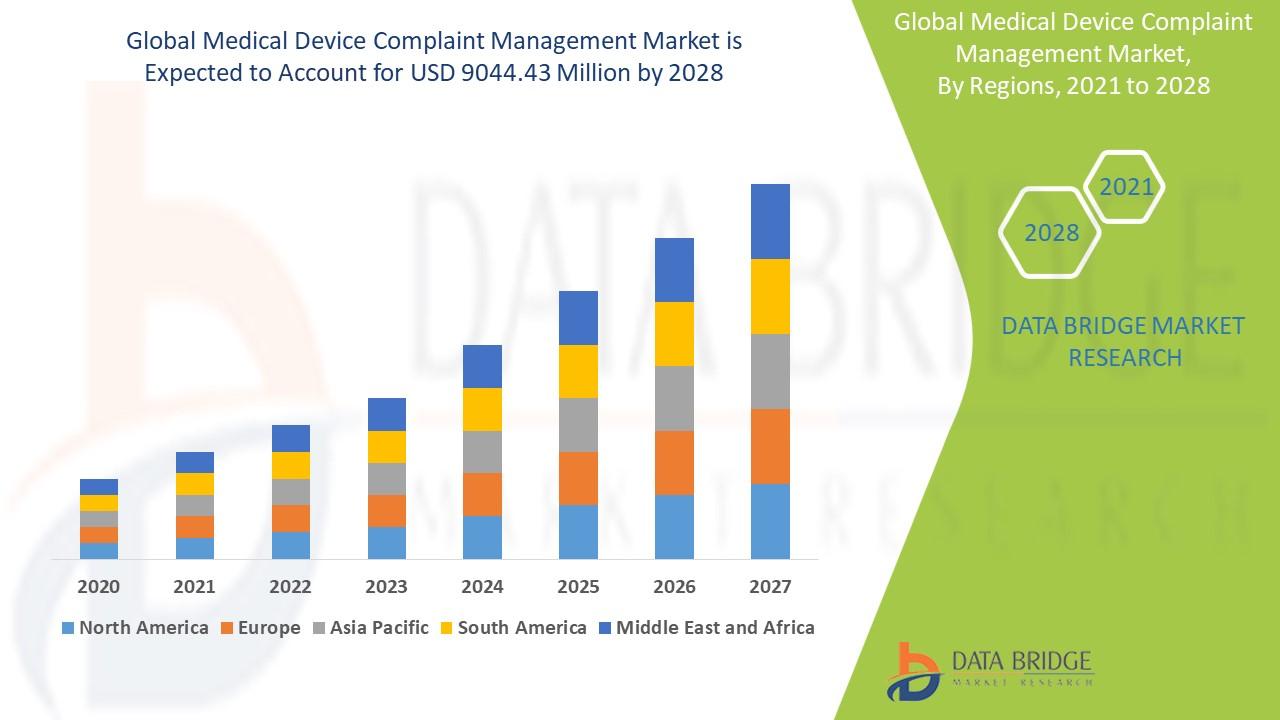
Data Bridge Market Research analyses the market to account to USD 9044.43 million by 2028 growing at a CAGR of 6.86% in the above-mentioned forecast period.
Market research studies stated in this Medical Device Complaint Management Marketreport are very thoughtful for the businesses which assist them with the better decision making and develop better strategies about production, marketing, sales and promotion. This Medical Device Complaint Management Market report brings together comprehensive industry analysis with exact estimates and forecasts that offers complete research solutions with maximum industry clarity. The report includes CAGR value fluctuations during the forecast period of 2018-2025 for the market. And to serve the clients best in the industry, a team of experts, skilled analysts, dynamic forecasters and knowledgeable researchers work meticulously while forming this report.
This Medical Device Complaint Management Market report is an ultimate source of information about the industry, important facts and figures, expert opinions, and the latest developments across the globe. The report studies various inhibitors as well as motivators of the market in both quantitative and qualitative manner so that users can have perfect information. The base year for calculation in the Medical Device Complaint Management Market report is considered as 2017 while the historic year is 2016 which will tell you how the Medical Device Complaint Management Market is going to perform in the forecast years. The Medical Device Complaint Management Market report introduces the basics of industry such as market definitions, classifications, applications and industry chain overview, after which it covers industry policies and plans, product specifications, manufacturing processes, cost structures and so on.
Find out what’s next for the Medical Device Complaint Management Market with exclusive insights and opportunities. Download full report:
https://www.databridgemarketresearch.com/reports/global-medical-device-complaint-management-market
Medical Device Complaint Management Market Dynamics
**Segments**
- By Type (Product Quality Complaints, Adverse Event Reporting)
- By Deployment (On-Premise, Cloud-Based)
- By End-User (Hospitals, Clinics, Ambulatory Surgical Centers, Others)
The global medical device complaint management market is segmented based on type, deployment, and end-user. In terms of type, the market is categorized into product quality complaints and adverse event reporting. Product quality complaints involve issues related to the manufacturing, packaging, or labeling of medical devices, while adverse event reporting deals with incidents where the use of a medical device has resulted in harm to a patient. By deployment, the market is divided into on-premise and cloud-based solutions, offering flexibility and scalability to healthcare organizations. Lastly, based on end-user, the market caters to hospitals, clinics, ambulatory surgical centers, and others, addressing the specific needs and requirements of different healthcare settings.
**Market Players**
- Sparta Systems, Inc.
- AssurX, Inc.
- MasterControl, Inc.
- Oracle
- Siemens AG
- Klarity Risk Management
- MD-QMS
- BSI Group
- QUMAS
- Pilgrim Quality Solutions
- Conceptual Innovations
- Xybion Corporation
- Flex Databases
- Creation Technologies
- ABS Consulting
Prominent players in the global medical device complaint management market include Sparta Systems, Inc., AssurX, Inc., MasterControl, Inc., Oracle, Siemens AG, Klarity Risk Management, MD-QMS, BSI Group, QUMAS, Pilgrim Quality Solutions, Conceptual Innovations, Xybion Corporation, Flex Databases, Creation Technologies, and ABS Consulting. These key market players offer innovative solutions and services to help healthcare organizations effectively manage complaints related to medical devices, ensuring regulatory compliance and patient safety. The competitive landscape is characterized by strategic partnerships, product launches, and technological advancements aimed at enhancing product offerings and expanding market reach.
The global medical device complaint management market is witnessing significant growth due to the increasing focus on patient safety and regulatory compliance in the healthcare industry. One key trend in the market is the rising adoption of cloud-based complaint management solutions, which offer enhanced accessibility, real-time data tracking, and cost-effective implementations for healthcare organizations. Cloud-based platforms provide scalability, secure data storage, and seamless integration with existing systems, driving their adoption across hospitals, clinics, and ambulatory surgical centers globally.
Another emerging trend in the market is the integration of advanced technologies such as artificial intelligence (AI) and machine learning (ML) to streamline complaint handling processes and improve decision-making capabilities. AI-powered algorithms can analyze large datasets to identify patterns, trends, and potential risks associated with medical device complaints, enabling proactive mitigation strategies and quality improvements. Moreover, the implementation of robotic process automation (RPA) in complaint management systems enhances operational efficiency, reduces manual errors, and accelerates response times in addressing customer feedback and regulatory requirements.
Furthermore, the market is witnessing a shift towards proactive risk management strategies, where healthcare organizations are investing in comprehensive complaint management solutions to identify potential issues early, prevent adverse events, and enhance overall patient outcomes. These proactive approaches involve continuous monitoring, trend analysis, and root cause investigations to drive continuous improvement in product quality and safety standards. Additionally, the focus on interoperability and data standardization is driving the integration of complaint management systems with electronic health records (EHRs) and other healthcare IT platforms, facilitating seamless data exchange and comprehensive patient care management.
Moreover, regulatory requirements and quality standards play a critical role in shaping the landscape of the medical device complaint management market. Increasing scrutiny from regulatory bodies such as the FDA and EMA on post-market surveillance and adverse event reporting necessitates robust complaint handling processes and documentation procedures for medical device manufacturers and healthcare providers. Compliance with standards such as ISO 13485 and FDA 21 CFR Part 820 is essential to ensure the safety, efficacy, and quality of medical devices, driving the demand for integrated complaint management solutions that streamline quality management systems and support regulatory audits.
In conclusion, the global medical device complaint management market is poised for substantial growth, driven by technological advancements, regulatory pressures, and the shifting focus towards patient-centric care. Key market players are continuously innovating and expanding their product portfolios to address the evolving needs of healthcare organizations and improve the overall quality and safety of medical devices. With the increasing adoption of cloud-based solutions, AI-powered analytics, and proactive risk management strategies, the market is set to witness further advancements in improving complaint handling processes, driving better patient outcomes, and ensuring compliance with stringent regulatory requirements.The global medical device complaint management market is highly dynamic and competitive, with a focus on enhancing patient safety, regulatory compliance, and overall quality of healthcare services. Key market players are investing in innovative solutions and strategic partnerships to stay ahead in the rapidly evolving landscape. One of the significant trends shaping the market is the increasing adoption of cloud-based complaint management systems, driven by the need for real-time data tracking, enhanced accessibility, and cost-effective implementations. Cloud platforms offer scalability and seamless integration with existing systems, making them a popular choice among healthcare providers worldwide.
Another crucial trend in the market is the integration of advanced technologies such as artificial intelligence (AI) and machine learning (ML) to streamline complaint handling processes and improve decision-making capabilities. AI-powered algorithms can analyze vast amounts of data to identify patterns and potential risks, enabling proactive risk mitigation and quality improvements. Additionally, the use of robotic process automation (RPA) is enhancing operational efficiency, reducing errors, and accelerating response times in addressing customer feedback and regulatory requirements. These technological advancements are reshaping how healthcare organizations manage medical device complaints, driving efficiency and improving patient outcomes.
Furthermore, there is a notable shift towards proactive risk management strategies in the market, with a focus on early identification of potential issues, prevention of adverse events, and continuous improvement in product quality and safety standards. Healthcare organizations are investing in comprehensive complaint management solutions that enable continuous monitoring, trend analysis, and root cause investigations to drive quality improvements. Moreover, the emphasis on interoperability and data standardization is promoting the integration of complaint management systems with electronic health records (EHRs) and other healthcare IT platforms to facilitate seamless data exchange and enhance patient care management.
Regulatory requirements and quality standards continue to play a significant role in shaping the market dynamics of medical device complaint management. Compliance with regulations such as ISO 13485 and FDA 21 CFR Part 820 is crucial for ensuring the safety, efficacy, and quality of medical devices, driving the demand for integrated complaint management solutions that streamline quality management systems and support regulatory audits. The increasing scrutiny from regulatory bodies on post-market surveillance and adverse event reporting is further driving the adoption of robust complaint handling processes and documentation procedures among medical device manufacturers and healthcare providers.
In conclusion, the global medical device complaint management market is witnessing robust growth driven by technological innovations, regulatory pressures, and a growing emphasis on patient safety and quality of care. Key market players are focused on developing advanced solutions, leveraging AI and cloud-based technologies, and promoting proactive risk management strategies to address the evolving needs of healthcare organizations effectively. As the market continues to evolve, the integration of advanced technologies and a proactive approach to risk management will be crucial for ensuring compliance with regulatory requirements, improving patient outcomes, and driving overall growth in the medical device complaint management market.
Track the company’s evolving market share
https://www.databridgemarketresearch.com/reports/global-medical-device-complaint-management-market/companies
Master List of Market Research Questions – Medical Device Complaint Management Market Focus
- What is the scope of the global Medical Device Complaint Management Market?
- What is the anticipated pace of growth for the Medical Device Complaint Management Market sector?
- What Medical Device Complaint Management Market segments are most profitable?
- Who are the powerhouses in the global Medical Device Complaint Management Market?
- What are the top-performing countries in the dataset for the Medical Device Complaint Management Market?
- What firms are ranked highest in revenue in Medical Device Complaint Management Market?
Browse More Reports:
Global Side Guard Door Beams Market
Global Silane Market
Global Silicon Alloys Market
Global Silicone Fabrics Market
Global Skin Packaging for Fresh Meat Market
Global Smart Contact Lenses for Disease Monitoring Market
Global Smart Shower Systems Market
Global Solar Powered Unmanned Aerial Vehicle (UAV) Market
Global Spear Phishing Market
Global Spinal Traction Market
Global Spine Biologics Market
Global Spintronics Market
Global Sport Caps and Closures Market
Global Squash Rackets Market
Global Starch Derivatives Market
U.S. Foot and Ankle Allografts Market
About Data Bridge Market Research:
An absolute way to forecast what the future holds is to comprehend the trend today!
Data Bridge Market Research set forth itself as an unconventional and neoteric market research and consulting firm with an unparalleled level of resilience and integrated approaches. We are determined to unearth the best market opportunities and foster efficient information for your business to thrive in the market. Data Bridge endeavors to provide appropriate solutions to the complex business challenges and initiates an effortless decision-making process. Data Bridge is an aftermath of sheer wisdom and experience which was formulated and framed in the year 2015 in Pune.
Contact Us:
Data Bridge Market Research
US: +1 614 591 3140
UK: +44 845 154 9652
APAC : +653 1251 975
Email:- corporatesales@databridgemarketresearch.com
"
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- ហ្គេម
- Gardening
- Health
- ផ្ទះ
- Literature
- Music
- Networking
- ផ្សេងៗ
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness

